What we do
Our group is motivated by the clinical need to create effective therapies for repairing the injured nervous system. We propose the brain tumor micro-environment as an excellent model system in which to uncover new methodologies for controlling and reprogramming the neural response to injury. We aim to uncover insights about how cancer promotes "tissue" growth so we can re-engineer those strategies as innovative regenerative biomaterials.
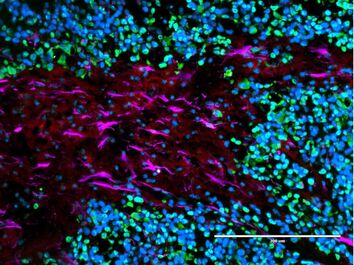
The onco-regenerative niche
Tissue responses to injury and cancer are quite opposite: Cancer is adept at training its environment to promote tumor growth, while neural cells after injury work to block off the lesion and hinder tissue growth. Our group is working to discover and isolate the myriad ways brain cancer cells train neighboring cells to take on pro-tumor, or pro-regenerative, states. We will then repurpose these signals in the form of implantable, therapeutic materials to aid spinal cord regeneration after injury and restore critical neural function.
Tissue responses to injury and cancer are quite opposite: Cancer is adept at training its environment to promote tumor growth, while neural cells after injury work to block off the lesion and hinder tissue growth. Our group is working to discover and isolate the myriad ways brain cancer cells train neighboring cells to take on pro-tumor, or pro-regenerative, states. We will then repurpose these signals in the form of implantable, therapeutic materials to aid spinal cord regeneration after injury and restore critical neural function.
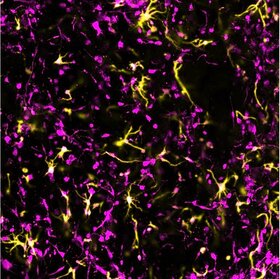
Microphysiological models of neural tissue
Our tissues have a complex and functionally-specific composition, and each individual element has its own contributions to health and disease. When researchers start to model these tissues in a dish, unfortunately, we often pretend using only one cell type in a static environment is sufficient. There is growing evidence that different elements (cells, matrix, biophysical forces) of the microenvironment can play important roles, especially in disease progression. We are working to expand this trend into the area of traumatic injury by developing new multicellular, dynamic models of the brain and spinal cord for probing and dissecting the distinct contributions of cells, matrix, and physical forces in the response to injury.
Our tissues have a complex and functionally-specific composition, and each individual element has its own contributions to health and disease. When researchers start to model these tissues in a dish, unfortunately, we often pretend using only one cell type in a static environment is sufficient. There is growing evidence that different elements (cells, matrix, biophysical forces) of the microenvironment can play important roles, especially in disease progression. We are working to expand this trend into the area of traumatic injury by developing new multicellular, dynamic models of the brain and spinal cord for probing and dissecting the distinct contributions of cells, matrix, and physical forces in the response to injury.
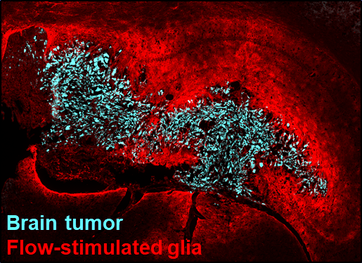
Fluid flow and shear effects in neurotrauma
What happens to the tissue immediately adjacent to an initial injury that causes it to later die, too? When the blood brain barrier is suddenly damaged, excess fluid accumulates at the site of injury. This again can be compared to cancer progression, during which leaky blood vessels - like those damaged by injury - create fluid build-up within the tumor. This fluid build-up grows in stark contrast with the surrounding tissue, forcing fluid out into the surroundings where it hits healthy cells and can cause detrimental reactions. Our team is investigating if similar fluid-driven changes occur after neural injury and strategies to protect those initially-unaffected healthy cells.
What happens to the tissue immediately adjacent to an initial injury that causes it to later die, too? When the blood brain barrier is suddenly damaged, excess fluid accumulates at the site of injury. This again can be compared to cancer progression, during which leaky blood vessels - like those damaged by injury - create fluid build-up within the tumor. This fluid build-up grows in stark contrast with the surrounding tissue, forcing fluid out into the surroundings where it hits healthy cells and can cause detrimental reactions. Our team is investigating if similar fluid-driven changes occur after neural injury and strategies to protect those initially-unaffected healthy cells.
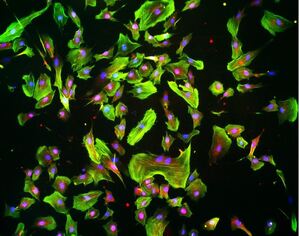
Fluid biomechanics and mechanotransduction
Related to our interests in fluid flow dynamics and effects in the nervous system, we are examining cellular- and molecular-level pathways involved in the mechanotransduction of fluid forces in neural cells. The objective is to better understand how neural cells sense and response to fluid flow and identify therapeutic targets to increase or decrease cellular sensing of these mechanical cues, depending on the context. For these analyses, we use microphysiological and microfluidic systems to precisely control the cellular and biophysical microenvironment in which to probe the mechanisms of neural cell flow response.
Related to our interests in fluid flow dynamics and effects in the nervous system, we are examining cellular- and molecular-level pathways involved in the mechanotransduction of fluid forces in neural cells. The objective is to better understand how neural cells sense and response to fluid flow and identify therapeutic targets to increase or decrease cellular sensing of these mechanical cues, depending on the context. For these analyses, we use microphysiological and microfluidic systems to precisely control the cellular and biophysical microenvironment in which to probe the mechanisms of neural cell flow response.
Funding


